
MANUFACTURER: RANDOX
MADE: UK
TYPE: QUALITY MANAGEMENT SOLUTIONS
QCMD is a world leading External Quality Assessment (EQA) / Proficiency Testing (PT) scheme, dedicated to improving the quality of molecular diagnostic assays used in the detection of infectious diseases. With an extensive database of over 15,000 participants in over 100 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics.
The aim of QCMD’s External Quality Assessment (EQA) programmes or schemes is to help monitor and improve laboratory quality by assessing a laboratory’s use of molecular testing for infectious diseases. The EQA schemes are both educational and regulatory in application and support continuous quality improvement, as well as assist laboratory accreditation / certification to ISO15189 or equivalent.
BENEFITS:
EXTENSIVE PROGRAMME OFFERING:
• Boasting the largest selection of molecular EQA programmes for infectious disease testing, you are sure to find what you’re looking for.
FREQUENCY:
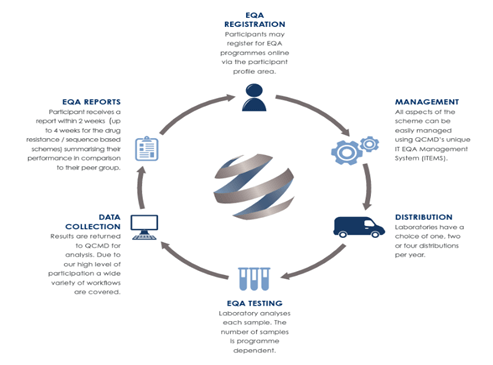
• Choose between one, two and four challenges* per year to suit your laboratory requirements. Reports are available within 2 weeks of the submission deadline (up to 4 weeks for the drug resistance / sequence based schemes), ensuring any corrective actions can be taken quickly.
HIGH QUALITY MATERIAL:
• The availability of whole pathogen samples in clinically relevant matrices mimics the performance of patient samples and ensures samples can be used to effectively monitor the performance of the entire testing process.
INTERNATIONAL ACCREDITATION:
• Where appropriate the EQA schemes are accredited to ISO 17043:2010 highlighting the superior quality and organisation of the QCMD scheme.
ONLINE EQA MANAGEMENT SYSTEM:
• IT EQA Management System (ITEMS) provides an online tool to easily manage all EQA activities from programme registration to submission of results and provision of EQA reports.
HIGH LEVEL OF PARTICIPATION:
• With over 15,000 participant registrations in more than 120 countries, peer groups are maximised, increasing statistical validity.
COMPREHENSIVE REPORTS:
• Individual reports are provided with each EQA challenge. In line with the requirements of ISO17043, they provide the laboratories with their results and performance assessment in relation to their EQA assessment group (peer review group). Supplementary reports which include scientific expert commentary may be provided at the end of the EQA cycle if appropriate.